The Case of the Translational Research
FEATURED PAPER
By Massimo Pirozzi
Rome, Italy
and
Dr. Lidia Strigari
Bologna, Italy
INTRODUCTION
The translational research “translates” research results in clinical results – mainly via the knowledge transfer and the adoption of appropriate protocols – and vice versa – mainly via the acquisition of patient information and feedback. This approach enables the setup, the maintenance, and the optimization of a harmonic flow that maximizes the synergies between the basic research, the laboratory tests, and the medical assistance, by performing clinical trials that are oriented to the generation of value and of benefits for the community in a large variety of healthcare-related basic issues, including medicines, specialized equipment, diagnoses, therapies and vaccines.
Since the translational research is based on evidences, which arise both from research and from clinical practice, it can be considered, in all respects, an application of evidence-based medicine. As we saw in a previous paper (Pirozzi and Strigari, 2020), in today’s world, both the extent and the complexity of healthcare projects need special approaches to reach efficacy and efficiency respecting ethical and other constraints, preserving the care to the person by achieving the satisfaction of stakeholders, and minimizing negative risks. The Evidence Based Medicine (EBM) takes into account the above factors by integrating best scientific evidence with both clinical expertise and patient values and expectations, while Project Management discipline can effectively support EBM projects in facing complexity with efficacy and efficiency. This paper briefly gives an overview about translational research, examines the peculiar characteristics of translational research projects, and proposes project management as an effective support to face complexity, to realize efficacy and efficiency, to satisfy stakeholder requirements and expectations, to target project success, and to reduce drastically the threats and the uncertainties.
THE TRANSLATIONAL RESEARCH
In general, the research is based on the researchers’ knowledge, enriched by the input of sources of evidence such as the Cochrane Library, PubMed, Medline, EMBASE, etc. For the purposes of formulating an appropriate question in the EBM process, the PICOT approach (Stillwell et al., 2010) is generally used to framing the clinical question of trial trough five components: P that corresponds to the population of interest, I that corresponds to the type of intervention or clinical action, C that corresponds to the comparison represented by “usual treatment” (or “nothing”), O that corresponds to all possible outcomes to be explored, T that corresponds to the time duration for intervention or to the outcome ascertainment time. Once the PICOT question is formulated, the search for evidence can start and then proceed step by step in generating value.
The Translational Research Value Chain (Fig.1) may be often characterized by a long way; indeed, several steps are mandatory to demonstrate the proof-of-principal (based on experiments conducted on cells and indicated as in vitro study) and the proof of concept (based on animal models that represent the first step of complexity), and, therefore, to verify these hypotheses into human-based clinical trials (phase I, II, and III).
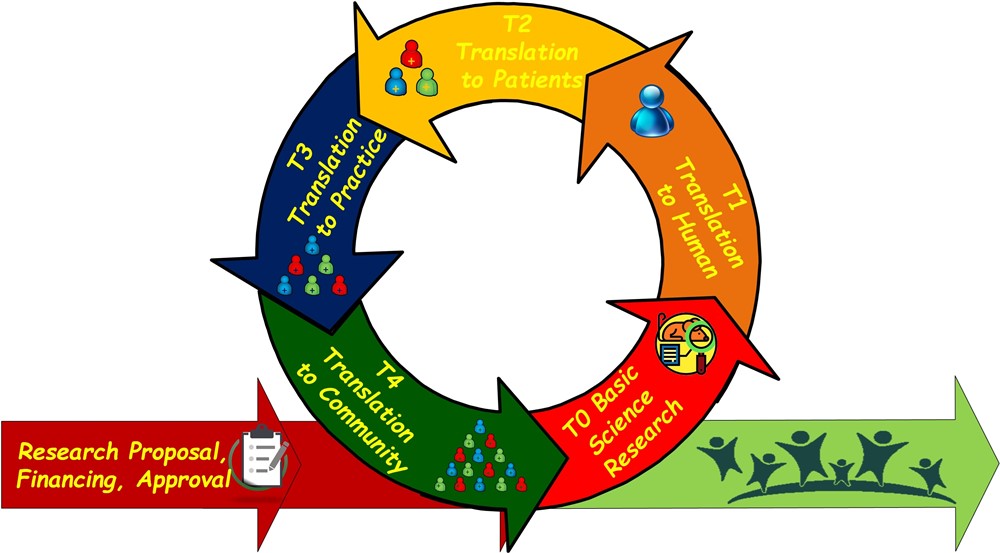
Fig.1 – The Translational Research Value Chain
Specifically, above iterative sequence moves from simple observational methods (which correspond to the steps T0, T1 and T2) to increasingly rigorous and systematic methodologies (which correspond to the steps T3 andT4). While the proof-of-principal – often indicated as in vitro study – is based on experiments conducted on cells, the proof of concept is based on animal models, and, then, it represents the first step of complexity to overcome in order to replicating the in vitro results also taking into consideration, for example, the immunity factors. The above approaches, which are indicated as T0 (preclinical & animal studies), focus on studying/defining pathobiology, mechanisms, targets, lead molecules, and also on an initial regulatory interaction, represent the first attempt of the translation from basic science to human studies, and are intrinsically subject to the risk of possible failure due to a “valley of death” (Seyhan et al. 2019) devoid of significant results.
The sequential – but potentially iterative – following steps are planned, implemented, and controlled to verify in clinical trials the hypotheses that were assessed in the preclinical models. The step indicated as T1 (Phase 1 of Clinical Trials, targeting safety, proof of mechanism and proof of concept, dose selection, …) includes the studies of new methods of diagnosis, treatment or prevention, and regards just a limited cohort of selected patients that could benefit from the relevant scientific discoveries, and who generally are not candidate to other treatments or strategies. The step indicated as T2 (Phases 2 and 3 of Clinical Trials, targeting proof of efficacy, safety) is conducted as a controlled study to provide the effective care, the benefit/risk profile, and the healthcare economic data improvement. Then, translation of new data into the clinical and health decision making are generally based on the subsequent steps indicated as T3 and T4. The T3 step (Phase 4 of Clinical Trials and Clinical Outcomes Research) aims to deliver recommended and timely care to right patients, post marketing safety, and new indications, while the step T4 (Population Level Outcomes Research) represents the final step, and aims to demonstrate the real benefits for the society by engaging in the trials a larger population.
More…
To read entire paper, click here
How to cite this paper: Pirozzi, M., Strigari, L. (2021). Project Management for Healthcare: The Case of the Translational Research; PM World Journal, Vol. X, Issue IV, April. Available online at https://pmworldlibrary.net/wp-content/uploads/2021/04/pmwj104-Apr2021-Pirozzi-Strigari-project-management-for-healthcare-translational-research.pdf
About the Authors

Massimo Pirozzi
Rome, Italy
![]()
Massimo Pirozzi, MSc cum laude, Electronic Engineering, University of Rome “La Sapienza”, Principal Consultant, Project Manager, and Educator. He is a Member of the Executive Board and of the Scientific Committee, and an Accredited Master Teacher, of the Istituto Italiano di Project Management (Italian Institute of Project Management). He is certified as a Professional Project Manager, as an Information Security Management Systems Lead Auditor, and as an International Mediator. He is a Researcher, a Lecturer, and an Author about Stakeholder Management, Relationship Management, and Complex Projects Management, and his papers have been published in U.S.A., in Italy, and also in Russia; in particular, he is the Author of the innovative Book “The Stakeholder Perspective: Relationship Management to enhance Project value and Success”, CRC Press, Taylor & Francis Group, Boca Raton (FL), U.S.A., October 2019. Due to the acknowledgement of his comments on stakeholder-related issues contained in Exposure Draft of The Standard for Project Management – 7th Edition, he will be also included in the list of Contributors and Reviewers of The PMBOK® Guide – Seventh Edition.
Massimo Pirozzi has a wide experience in managing large and complex projects, programs, and portfolios in national and international contexts, and in managing business relations with public and private organizations, including multinational companies, small and medium-sized enterprises, research institutes, and non-profit organizations. He worked successfully in several sectors, including Defense, Security, Health, Education, Engineering, Logistics, Cultural Heritage, Transport, Gaming, Services to Citizens, Consulting, and Web. He was also, for many years, a Top Manager in ICT Industry, and an Adjunct Professor in Organizational Psychology. He is registered as an Expert both of the European Commission, and of Italian Public Administrations.
Massimo Pirozzi is an Accomplished Author and the International Correspondent in Italy of PM World Journal. He received two 2019 PM World Journal Editor’s Choice Awards for his featured paper “Stakeholders, Who Are They?”, and for his report from Italy titled “PM Expo® and PM Maturity Model ISIPM-Prado®”. He received also the 2018 PM World Journal Editor’s Choice Award for his featured paper “The Stakeholder Management Perspective to Increase the Success Rate of Complex Projects”.
Massimo can be contacted at max.pirozzi@gmail.com.

Dr. Lidia Strigari
Bologna, Italy
![]()
Lidia Strigari, MSc cum laude in Physics, Doctor of Medical Physics, PhD in Advanced Technologies in Biomedicine, Doctor’s Degrees in Physical Basis and Technology in the field of hadron-therapy and stereotactic radiotherapy, in Statistics in Biomedical Science, in Economy and Management in Healthcare, holds a Certification in Project Management released by the Istituto Italiano di Project Management (Italian Institute of Project Management).
Lidia Strigari is presently Head of Department of Medical Physics and Expert Systems at the St. Orsola University Hospital and Research Center in Bologna. She has previously been the Head of the Laboratory of Medical Physics and Expert Systems at the IRCCS Regina Elena National Cancer Institute (IRE-IFO). She is Associate Professor of the Medical Physics Post-graduated Specialization School at the University “Tor Vergata” of Rome, and she is Associate Professor of the Medical Physics at the Medical Physics, radiotherapy and Nuclear Medicine Specialization School at the University of Bologna.
Lidia Strigari is a well-recognized clinical researcher both at national and international levels, who focused her research interests on the fields of dosimetry, radiobiological models, different radiation treatment modalities, systemic therapy and diagnostics. She has been also involved in phase II and phase III clinical trials on moderate hypofractionation and dose escalation for prostate and breast cancer, thus balancing and complementing her knowledge with translational research issues. Publication record – with more than 170 articles published in indexed/peer-reviewed journals (h-index=25), several book chapters – and two patents (PCT/IT2014/000147, PCT/IB2016/052002) evidence her strong expertise in all these fields of research. She is and has been a principal investigator of several international and national projects, in collaboration with important Research Centers and Institutions, receiving and managing funds from Ministry of Health, AIRC, INAIL, Lazio Region, and NATO. She has been a member of “dosimetry committee” of European Association of Nuclear Medicine Dosimetry Committee, as an expert of radiobiology. She is a member of “EANM Radiobiology Working Group” and of the International Commission on Radiation Units & Measurements (ICRU) Report Committee 31 – “Treatment Planning for Radiopharmaceutical Therapy”.
Lidia Strigari can be contacted at lidia.strigari@aosp.bo.it.









